Details of the Drug
General Information of Drug (ID: DMQ4HIN)
| Drug Name |
Cabergoline
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cabaser; Cabaseril; Cabergolina; Cabergolinum; Dostinex; Galastop; Sogilen; Cabergolina [Spanish]; Cabergolinum [Latin]; CG-101; Cabaser (TN); Dostinex (TN); FCE-21336; Cabergoline [USAN:BAN:INN]; Cabergoline (JAN/USAN/INN); (8R)-6-allyl-N-[3-(dimethylamino)propyl]-N-(ethylcarbamoyl)ergoline-8-carboxamide; (8beta)-N-[3-(dimethylamino)propyl]-N-(ethylcarbamoyl)-6-(prop-2-en-1-yl)ergoline-8-carboxamide; (8beta)-N-[3-(dimethylamino)propyl]-N-[(ethylamino)carbonyl]-6-(2-propenyl)-ergoline-8-carboxamide; (8beta)-N-[3-(dimethylamino)propyl]-N-[(ethylamino)carbonyl]-6-prop-2-en-1-ylergoline-8-carboxamide; 1-((6-Allylergolin-8beta-yl)carbonyl)-1-(3-(dimethylamino)propyl)-3-ethylurea; 1-[(6-allylergoline-8beta-yl)carbonyl]-1-[3-(dimethylamino)propyl]-3-ethylurea; 1-ethyl-2-(3'-dimethylaminopropyl)-3-(6'-allylergoline-8'-beta-carbonyl)urea diphosphate; 1-ethyl-3-(3'-dimethylamionpropyl)-2-(6'-allylergoline-8'beta-carbonyl)urea
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
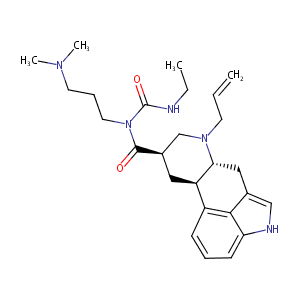 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 451.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hyperprolactinaemia | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A60.1 | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Cabergoline (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 37). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Role of D1 and D2 receptors in the regulation of voluntary movements. Bull Exp Biol Med. 2008 Jul;146(1):14-7. | ||||
| 7 | Polymorphisms of the drug transporter gene ABCB1 predict side effects of treatment with cabergoline in patients with PRL adenomas. Eur J Endocrinol. 2012 Sep;167(3):327-35. | ||||
| 8 | Receptor-binding and pharmacokinetic properties of dopaminergic agonists. Curr Top Med Chem. 2008;8(12):1049-67. | ||||
| 9 | Atractylon, a novel dopamine 2 receptor agonist, ameliorates Parkinsonian like motor dysfunctions in MPTP-induced mice. Neurotoxicology. 2022 Mar;89:121-126. doi: 10.1016/j.neuro.2022.01.010. Epub 2022 Jan 31. | ||||
| 10 | Massive reduction of tumour load and normalisation of hyperprolactinaemia after high dose cabergoline in metastasised prolactinoma causing thoracic syringomyelia. J Neurol Neurosurg Psychiatry. 2004 Oct;75(10):1489-91. doi: 10.1136/jnnp.2003.028118. | ||||
| 11 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 12 | Mims RB, Scott CL, Modebe O, Bethune JE "Inhibition of L-dopa-induced growth hormone stimulation by pyridoxine and chlorpromazine." J Clin Endocrinol Metab 40 (1975): 256-9. [PMID: 1117978] | ||||
| 13 | Ausband SC, Goodman PE "An unusual case of clarithromycin associated ergotism." J Emerg Med 4 (2001): 411-3. [PMID: 11728770] | ||||
| 14 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 15 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 16 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 17 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 18 | Hansen BS, Dam M, Brandt J, et al "Influence of dextropropoxyphene on steady state serum levels and protein binding of three anti-epileptic drugs in man." Acta Neurol Scand 61 (1980): 357-67. [PMID: 6998251] | ||||
| 19 | Product Information. Zanaflex (tizanidine). Acorda Therapeutics, Hawthorne, NY. | ||||
| 20 | Product Information. Norprolac (quinagolide). Ferring Inc, North York, IA. | ||||
